Declining Capacity
Energy storage in a battery can conceptually be divided into three imaginary segments of the available energy,the empty zonethat can be refilled, and the unusable part (rock content). Figure 1 illustrates these three sections.
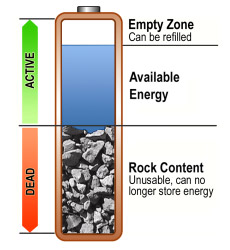 | Figure 1: Aging battery Batteries begin fading from the day they are manufactured.A new battery should deliver 100 percent capacity; most packs in use operate at less. Courtesy of Cadex |
Although the manufacturer specifies the runtime of portable equipment based on a battery performing at 100 percent, most packs in the field operate at less capacity. As time goes on, the performance declines further and the battery gets smaller in terms of holding capacity. A pack should be replaced when the capacity drops to 80 percent. This is only 20 percent down from 100 percent, and the end-of-life threshold may vary according to application and company policy.
Rising Internal Resistance
High capacity has limited use if the battery is unable to deliver the stored energy effectively. To bring the power out, the battery needs low internal resistance. Measured in milliohms (mW), resistance is the gatekeeper of the battery; the lower the value, the less restriction the pack encounters. This is especially important with heavy loads and high current pulses, as elevated resistance causes the voltage to collapse and trigger an earlyshutdown. The device turns off and valuable energy is left behind. Figure 2 illustrates batteries with low and high internal resistance as free-flowing and restricted taps. 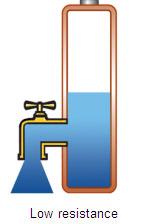 | 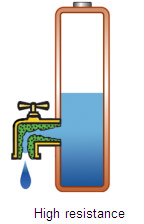 | Figure 2: Effects of internal battery resistance A battery with low internal resistance delivers high current on demand. High resistance causes the battery voltage to collapse. The equipment cuts off, leaving energy behind. Courtesy of Cadex |
Alkaline, carbon-zinc and other primary batteries have relatively high internal resistance, and this relegates their use to low-current applications such as flashlights, remote controls, portable entertainment devices and kitchen clocks. As the batteries discharge, the resistance increases further. This explains why regular alkaline cells have a relatively short runtime in digital cameras. The high internal resistance limits most primary batteries to “soft” applications, and using them to drive power tools that draw high amperage is unthinkable.
Figures 3, 4 and 5 reflect the talk-time of cellular phones with pulsed discharge loads of 1C, 2C and 3C, which GSM and CDMA demand. All batteries tested are similar in size and have capacities of 113%, 94% and 107% respectively, when checked with a battery analyzer on a DC discharge. The three graphs clearly demonstrate the importance of low internal resistance, which varies from a low 155mΩto a moderate 320mΩ, to a high 778mΩ respectively.
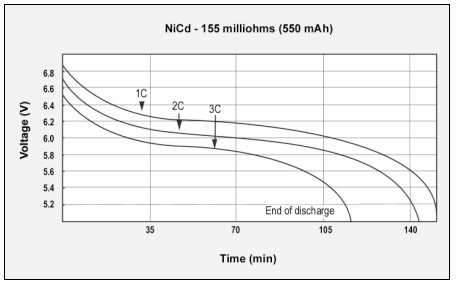
Figure 3: GSM discharge pulses at 1, 2, and 3C with resulting talk-time
The capacity of the NiCd battery is 113%; the internal resistance is 155mΩ.
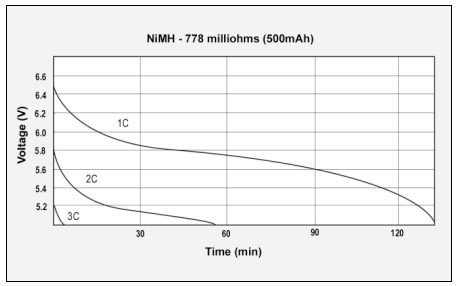
Figure 4: GSM discharge pulses at 1, 2, and 3C with resulting talk-time
The capacity of the NiMH battery is 94%, the internal resistance is 320mΩ.
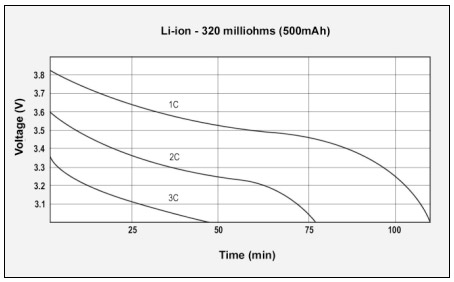
Figure 5: GSM discharge pulses at 1, 2, and 3C with resulting talk-time
The capacity of the Li-ion battery is 107%; the internal resistance is 778mΩ.
All three figures courtesy of Cadex
Notes: The above tests were done on cellular phone batteries before lithium-ion took over as the leading battery type for this application. The internal resistance of a modern cellular battery is between 150 and 350mΩ.
The maximum discharge pulse current of GSM is 2.5 amperes. When drawn from an 800mAh pack, this represents a 3C discharge, or three times the rated current.
Elevated Self-discharge
All batteries are affected by self-discharge. Self-discharge is not a manufacturing defect per se, although poor manufacturing practices and improper handling can promote the problem. The amount of electrical leakage varies with chemistry, and primary cells, such as lithium and alkaline, are among the best in retaining the energy. Nickel-based rechargeable systems, in comparison, leak the most and need recharging if the toshiba pa3399u-1bas battery has not been used for a few days. High-performance nickel-based batteries are subject to higher self-discharge than the standard versions with lower energy densities. Figure 6 illustrates in the form of leaking fluids the self-discharge of a battery.
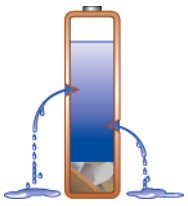 | Figure 6: Effects of high self-discharge Self-discharge increases with age, cycling and elevated temperature. Discard a battery if the self-discharge reaches 30 percent in 24 hours. Courtesy of Cadex |
The energy loss is asymptotical, meaning that the self-discharge is highest right after charge and then tapers off. Nickel-based batteries lose 10 to 15 percent of their capacity in the first 24 hours after charge, then 10 to 15 percent per month. Figure 7 shows the typical loss of a nickel-based battery while in storage.
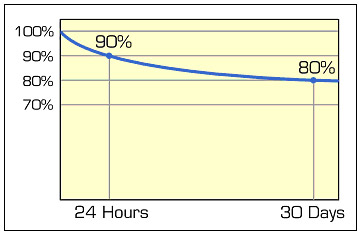 | Figure 7: Self-discharge as a function of time The discharge is highest right after charge and tapers off. The graph shows self-discharge of a nickel-based battery. Lead- and lithium-based system have a lower self-discharge. Courtesy of Cadex |
One of the best batteries in terms of self-discharge is lead acid; it loses only five percent per month. This chemistry also has the lowest specific energy and is ill suited for portable use. Lithium-ion self-discharges about five percent in the first 24 hours and 1 to 2 percent thereafter. The need for the protection circuit increases the discharge by another three percent per month.
The self-discharge on all battery chemistries increases at higher temperatures and the rate typically doubles with every 10°C (18°F). A noticeable energy loss occurs if a battery is left in a hot vehicle. Cycling and aging also increase self-discharge. Nickel-metal-hydride is good for 300-400 cycles, whereas the standard nickel-cadmium lasts over 1,000 cycles before elevated self-discharge starts interfering with performance. The self-discharge on an older nickel-based battery can get so bad that the pack loses its energy mainly through leakage rather than normal use during the day. Discard a dell latitude d620 battery if the self-discharge reaches 30 percent in 24 hours.
The self-discharge of Li-ion is reasonably steady throughout the service life and does not increase noticeably with age, unless there is a cell anomaly caused by separator damage when microscopic metal particles group together. Improved manufacturing methods have minimized this problem on newer batteries.Table 8 reveals the self-discharge rate per month at various temperatures and state-of-charge conditions.
| Charge condition | 0°C (32°F) | 25°C (77°F) | 60°C (140°F) |
| Full charge 40–60% charge | 6% 2% | 20% 4% | 35% 15% |
Self-discharge increases with rising temperature and higher SoC.
Once present, the high self-discharge of a flooded lead acid battery cannot be reversed. Factors leading to this failure are sludge buildup in the sediment trap on the bottom of the container. The sludge is semi-conductive, and when substance reaches to the plates a soft short occurs. On nickel-based batteries, a weakened or damaged separator is the cause of high self-discharge. Contributing factors are crystalline formation (memory), permitting the battery to “cook” in the charger or exposing it to repeated deep discharge cycles. A faulty separator also increases the self-discharge in lithium-ion batteries. In extreme cases, heat generated by the electrical leak further weakens the damaged separator. This can lead to a thermal breakdown.
Premature Voltage Cut-off
Not all stored battery energy can or should be used on discharge, and some reserve is almost always left behind when the equipment cuts off. There are several reasons for this.Most cell phones, laptops and other portable devices turn off when the lithium-ion battery reaches 3V/cell on discharge. The manufacturers choose this relatively high voltage threshold to allow for some self-discharge while in storage, giving a grace period before the protection circuit opens at about 2.5V/cell.
A hybrid battery on a car never fully discharges and operates on a state-of-charge of 20 to 80 percent. This is the most effective working bandwidth of the battery, and staying within this range delivers the longest service life. A deep discharge with a full recharge causes undue stress to any battery, including Li-ion. Nickel-based batteries are similar, and because of reduced charge acceptance and heat buildup above the 80 percent SoC, the batteries are seldom fully charged. The emphasis on an electric powertrain is on maximizing service life rather than optimizing runtime (as is the case with consumer products).
Power tools and medical devices that draw high currents push the battery voltage to an early cut-off. This is especially true if one of the cells has a high internal resistance, or when the battery is operating at cold temperatures. These batteries may still have ample capacity left after the “cut-off” and when discharging at moderate load, a acer travelmate 6592 battery analyzer may read a residual capacity of 30 percent. Figure 9 illustrates the cut-off voltage graphically.
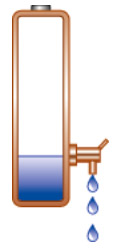 | Figure 9: Illustration of equipment with high cut-off voltage Portable devices do not utilize all available battery power and leave some energy behind. Courtesy of Cadex |




No comments:
Post a Comment