Battery-powered Vehicles
Batteries for propulsion systems have been in use for over 100 years, and today electric powertrains drive robots, bicycles, wheelchairs, golf cars, forklifts, EVs and underwater vessels. This power source has one thing in common; it is pollution-free and can be used indoors and underwater. For reasons of battery size, propulsion systems for heavy outdoor equipment such as earthmoving machines, non-electrified trains, aircraft and large ships must continue to rely on fossil fuel.
Most electric bicycles in developing countries run on lead acid batteries. While inexpensive, lead acid for deep-cycle use is ill suited and the batteries last for only 9 to 12 months on a daily commute. Nickel- or lithium-based batteries with twice and three-times the specific energy offer better cycle performance and shorter charge times but are expensive. While lead acid comes in at less than $100 a pack, a nickel-based battery costs $400–500, and a high-end Li-ion goes for $800–1,200. At a capacity of 280–480Wh, the battery has a range of 20–40km. With flat terrain and good wind conditions, the battery power with 70 percent pedal assist is only 1kW per kilometer (1.6kW per mile). Uphill propulsion consumes up to 10Wh/km (16kW per mile).
The battery cost dictates the developing world to choose lead acid. If the commuter had a bit more money he would likely buy a motorcycle. In the wealthy West, bicycle owners use their bicycles more as a form of recreation than a necessity. They have the means to go for a better battery, and advanced e-bikes with NiMH and
Li-ion batteries sell for several thousand dollars. Europe is leading in the up-scale electrical bicycle and the trend is spreading.
Wheelchairs, scooters and golf cars use mostly lead acid batteries. Even though heavy, lead acid works reasonably well and alternative chemistries would be too expensive. While wheelchair batteries tend to have a short service life span of about two years, a similar battery in a golf car can last for 4 to 5 years. This, I believe, is due to charging practices. The lead acid battery needs a fully saturated charge of 14–16 hours to prevent sulfation, and the time is not always available for the daily wheelchair user who may only charge the battery for eight hours while asleep. Golf car batteries, on the other hand, typically receive the needed 14–16 hours in a full overnight charge.
Ever since the starter motor was invented in 1912, lead acid batteries began cranking engines and providing power for lighting and ignition. Low cost and high current loading make lead acid an almost perfect candidate for starter applications. A typical starter battery has about 720 watts, and one of its unique qualities is good cranking ability even when the capacity fades to 25 percent or less.
Hybrids, plug-ins and electric vehicles use larger batteries, and Figure 1 compares the battery sizes. While the hybrid can get by with a battery twice the size of a starter battery, plug-in vehicles carry batteries in the 5–15kWh range, and the pure EV includes a monster battery ranging from 20 to 50kWh. Read more about the Electric Vehicle.
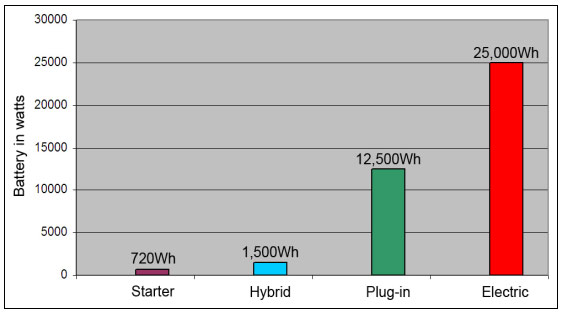 Figure 1: Typical battery wattages of vehicle batteries.
Figure 1: Typical battery wattages of vehicle batteries. While starter and hybrid batteries are tolerant to capacity fade, a weak EV battery travels shorter distances.
Courtesy of Cadex
The automotive industry is very conservative, and the choice of
toshiba pa3819u-1brs battery for most modern electric powertrains is lithium-ion with a nickel-manganese-cobalt mix (NMC). These cells provide stable service for many years and have low self-discharge, even when aging. NMC is also a desirable battery for power tools. Another strong candidate is Li-phosphate, a battery that delivers the best cycle life and is safe but has higher self-discharge than NMC. This complicates battery management, especially if the cells age differently.
University students converting an old Volkswagen Beetle to an electric powertrain to drive around the globe would shop for a lower-priced alternative and likely find a source in China. China offers Li-ion mono-blocks in 40–800Ah sizes at attractive prices. These batteries work well for less demanding applications and are great for experimental uses. There is, however, concern about safety and reliability when placed into the hands of common consumers.
The need of a battery management system becomes evident. It prevents any cell from exceeding 4.25V/cell on charge and dropping below 3.00V on discharge. As the cells age, cell capacities diverge and this affects charge and discharge times. On charge, a weak cell reaches full charge first, and without limit the voltage would rise further. On discharge, the weak cell discharges first and needs protection from voltage depletion. Weak cells are at a disadvantage; they get stressed the most and lose capacity quicker than the strong cells in a pack.
Forklifts use mostly lead acid batteries. Here, the weight is of little concern, however, long charging times is a disadvantage for warehouses operating 24 hours a day. This limits the fleet operation to only one shift. Fuel cell makers are gaining inroads by offering charging while the vehicle is in use. The addition of a fuel cell serving as onboard charger reduces battery size, but eliminating the battery entirely is not possible. The fuel cell has poor response characteristics on power demand and lacks the needed power bandwidth; the
toshiba pa3450u-1brs battery fills in for these shortcomings. Read more about the fuel cell.
The heavier the wheeled application, the more difficult it becomes to use batteries as the main powertrain. This does not prevent engineers from looking into alternate power sources to replace polluting diesel engines. One application under consideration is to use batteries for the Automatic Guided Vehicle (AGV) systems at ship ports, but battery size and charging times make this unfeasible. AGVs run 24 hours a day and the vehicles cannot be removed for lengthy charging. An automated battery exchange is being considered by removing the 10-ton, 300kWh lead acid battery from the vehicle and putting it on charge. Cost and impracticality may limit such an approach.
A German firm looked at using lithium-ion batteries for AGVs to speed up charging and reduce weight. While many smaller applications have switched to this new battery system, Li-ion is not yet ready for very large applications; the cost is prohibitive and the safety of such systems remains in issue.
On large-scale applications, batteries continue to have a hard time competing with fossil fuel in terms of specific energy. While a modern Li-ion battery produces about 120Wh/kg of energy, the net calorific value (NCV) of fossil fuel is 12,000Wh/kg, or one liter, an energy that is one hundred times higher. Even at a low efficiency of 25 percent, which an IC engine delivers, batteries don’t come close to this delivery of power.
Will Li-ion advance to take this spot? Perhaps not in our lifetime. Even if modern technology enabled large energy storage devices, charging these mega-batteries in an hour could dim a city. Replacing large diesel engines with batteries does not make commercial sense for now, nor can the fuel cell fill the spot. We need to breathe diesel-polluted air a little longer.
Batteries for Aviation
The duty of batteries on board aircraft is to run navigation and emergency systems when the Auxiliary Power Unit (APU) is off or if an emergency occurs. In the event of an engine failure, the batteries must supply energy from 30 minutes to three hours. Each aircraft must also have enough
toshiba pa3594u-1brs battery power to facilitate a safe landing.
Starting a large aircraft involves two stages. Most commercial jet aircraft use flooded nickel-cadmium to first engage the APU located at the tail end of a plane. The APU takes significantly longer to start and requires more energy than cranking the reciprocating engine in a vehicle. The spooling speed of the APU must be sufficiently high to attain compression for self-sustained ignition. This takes about 15 seconds and consumes 15kW of energy. Once running, an air compressor or hydraulic pump jumpstarts the large jet engines. On smaller aircraft, the battery must spool each engine for 25–40 seconds at high current. This puts far more stress on a battery than starting a car, and the batteries must be built accordingly.
Smaller aircraft may use a sealed lead-tin battery that is heavier than NiCd but has lower maintenance. The 12 and 24V aviation batteries are rated in IPP and IPR rather than CCA, as is common in the auto industry. Modern jet fighters spool the engines with lithium-ion batteries.
Durability and good performance at low temperature are the main reasons for the continued use of nickel-cadmium batteries in aviation. Most are flooded and require high maintenance that includes exercising to eliminate memory. The service consists of totally discharging the battery and placing a shortening strap across each cell for 24 hours. Each
toshiba satellite a200 battery is also checked for capacity with a battery analyzer.
Although aircraft carry many batteries aboard, their sole purpose is to provide starting and backup power. No passenger would dare fly to Europe or Asia on battery power alone. One can clearly see the limitations of batteries for large engines, and we need to rely on fossil fuel a little bit longer. (Let’s not give away this precious nonrenewable resource too cheaply by allowing people to squander the oil, especially if alternative energy storage devices, i.e. the batteries, can be used for ground transportation.)
Batteries for Aerospace
Early satellites used exclusively NiCd batteries. This, by the way, exposed the “memory” phenomenon in that NiCd could remember the amount of energy that was used on a tightly regulated discharge schedule. If the discharge lasted longer than normal, the battery would suffer a mysterious voltage drop. Today, most modern satellites, including the Hubble, use nickel-hydrogen cells. One of the enduring qualities of nickel-hydrogen is long cycle life. To optimize longevity, engineers over-design the batteries to achieve a small depth of discharge of only 6 to 10 percent.
High price and large size limit nickel-hydrogen batteries for satellite applications. Each cell has the appearance of a small steam engine and costs about a thousand dollars. These batteries are specially made for the application.
Satellites designed with a life span of five years or less often use lithium-ion. A new breed of Li-ion is being developed that promises to last 18 years. This would satisfy most satellite requirements and replace the heavier nickel-based systems. The battery in development is a large 140Ah cell. Li-ion is lighter in weight, is easier to charge and has a lower self-discharge than the nickel-based
toshiba satellite a350 battery systems of old. Furthermore, industrial versions of Li-ion promise to exceed the life span of nickel.