Most portable batteries are rated at 1C, meaning that a 1,000mAh battery that is discharged at 1C rate should under ideal conditions provide a current of 1,000mA for one hour. The same battery discharging at 0.5C would provide 500mA for two hours, and at 2C, the 1,000mAh battery would deliver 2,000mA for 30 minutes. 1C is also known as a one-hour discharge; a 0.5C is a two-hour, and a 2C is a half-hour discharge.
The battery capacity, or the amount of energy a battery can hold, can be measured with a battery analyzer. The analyzer discharges the battery at a calibrated current while measuring the time it takes to reach the end-of-discharge voltage. An instrument displaying the results in percentage of the nominal rating would show 100 percent if a 1,000mAh test battery could provide 1,000mA for one hour. If the discharge lasts for 30 minutes before reaching the end-of-discharge cut-off voltage, then the battery has a capacity of 50 percent. A new battery is sometimes overrated and can produce more than 100 percent capacity; others are underrated and never reach 100 percent even after priming.
When discharging a battery with a battery analyzer capable of applying different C‑rates, a higher C‑rate will produce a lower capacity reading and vice versa. By discharging the 1,000mAh battery at the faster 2C, or 2,000mA, the battery should ideally deliver the full capacity in 30 minutes. The sum should be the same as with a slower discharge since the identical amount of energy is being dispensed, only over a shorter time. In reality, internal resistance turns some of the energy into heat and lowers the resulting capacity to about 95 percent or less. Discharging the same battery at 0.5C, or 500mA over two hours, will likely increase the capacity to above 100 percent.
To obtain a reasonably good capacity reading, manufacturers commonly rate lead acid at 0.05C, or a 20-hour discharge. Even at this slow discharge rate, the battery seldom attains a 100 percent capacity. Manufacturers provide capacity offsets to adjust for the discrepancies in capacity if discharged at a higher C‑rate than specified. Figure 1 illustrates the discharge times of a lead acid battery at various loads as expressed in C-rate.
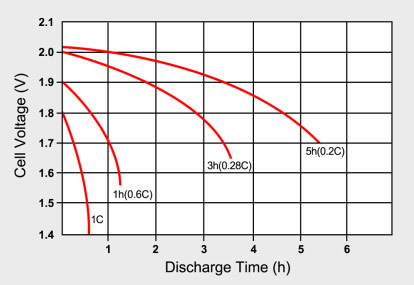
Figure 1: Typical discharge curves of lead acid as a function of C-rate
Smaller batteries are rated at a 1C discharge rate. Due to sluggish behavior, lead acid is rated at 0.2C (5h) and 0.05C (20h).
While lead- and nickel-based batteries can be discharged at a high rate, a safety circuit prevents Li-ion with cobalt cathodes from discharging above 1C. Manganese and phosphate can tolerate discharge rates of up to 10C and the current threshold is set higher accordingly.




No comments:
Post a Comment